Desentum: imagine a world without allergies
Seed Round in 2016
Desentum develops the next generation of immunotherapy to fight allergies. Whereas current approaches use purified natural allergens, Desentum develops modified hypoallergens, currently showing excellent results in the laboratory.



THE BUSINESS
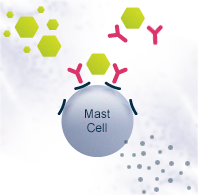
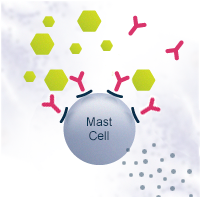
Allergy is a disorder of the immune system where a normally harmless environmental substance – such as food, pollen or animal dander – causes an allergic reaction. In Europe, 150 million people already suffer from allergies and the number is increasing rapidly. Allergies cause social and economic burden such as health care costs, missed school and work days and impact on the daily lives of the patients.
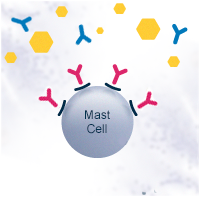
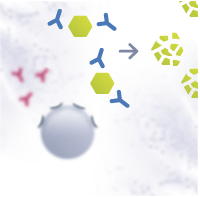
Allergies are generally managed by medication that alleviates the symptoms. The most common medications are antihistamines and corticosteroids. Immunotherapy is the only treatment currently known that affects the mechanism of allergy. It re-educates the immune system to tolerate the allergen, decreasing the need for medication. Immunotherapy can be administered as injections or sublingual tablets or drops, and the treatment usually takes a few years. The novel immunotherapeutic products that are under development aim for speeding up the treatment as well as improving safety, efficacy and convenience.
For the past 10 years, research groups of VTT Technical Research Centre of Finland, the University of Eastern Finland and Desentum have been using DNA techniques to modify allergens and improve immunotherapy.
THE ENTREPRENEURS
As a scientist and entrepreneur, Pekka Mattila has always worked at the forefront of biotechnology. For over 23 years he grew Finnzymes Oy to eventually sell the company to Fisher Scientific in 2011. He serves on the boards of several biotechnology companies since.
Prof. Juha Rouvinen of the university of Eastern Finland is known for his work on 3-dimensional structural identification of allergens.
THE PARTNERSHIP
Adriaan Hart de Ruijter led the seed round and joined the board in 2016. ‘Adriaan supports us with the clinical development experience we need so much right now, going into clinical trials with our lead product’, according to Pekka Mattila.
